Abstract
Background: T cell senescence is a physiological process typically associated with aging. In addition, lymphopenia and chronic immune activation can result in T cell senescence. CD57, a member of the N-CAM family initially described as a natural killer cell marker, has been reported as a marker of human senescent CD8 T cells. Percentages of CD57+ CD8 T cells increase during aging as well as during chronic viral infections. Early studies have reported increased proportions of CD57-expressing CD8 T cells after autologous and allogeneic hematopoietic stem cell transplantation (HSCT). Whether these cells can be considered a counterpart of the senescent population found during aging is at present unknown. More importantly, whether the expansion of CD57+ CD8 T cells is associated with impaired immunocompetence after allogeneic HSCT remains to be investigated.
Materials and Methods: With written informed consent, peripheral blood mononuclear cells were collected from healthy controls (HC, n=21) and patients undergoing allogeneic HSCT (n=115) at Geneva University Hospitals. Proportions of CD57+ CD8 T cells as well as phenotypic and functional characteristics of CD57+ CD8 T cells were assessed by flow cytometry. Virus-specific CD8 T cells were identified by flow cytometry based on IFNg and/or TNFa intra-cytoplasmic expression after 6h in vitro stimulation with peptides derived from CMV, EBV, HHV6, BKV and Adenovirus. Torque Teno Virus (TTV) replication was quantified by quantitative PCR as previously described (Pradier et al., Front Immunol 2020; doi: 10.3389/fimmu.2020.00998).
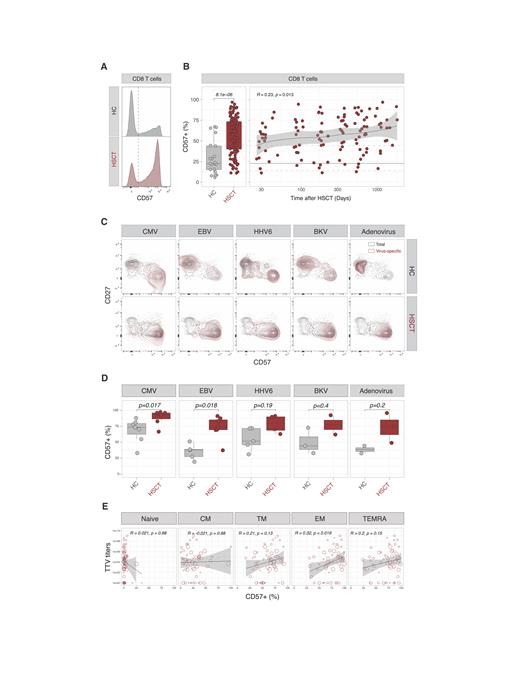
Results: CD8 T cells recovered from allogeneic HSCT displayed significantly higher proportions of CD57+ cells compared with healthy controls (HC, median 23% [range 6%-67%]; HSCT 58% [11%-97%]; p= 8.1e−06; Figure 1A-B). Such a difference was detected at early time points after transplantation and further increased at later time points (Figure 1B). After taking into account T cell subsets heterogeneity, we observed higher proportions of CD57+ cells in CD45RA- CCR7+ CD27+ central memory (CM; p= 0.00057), CD45RA- CCR7- CD27+ transitional memory (TM; p=1.1e-05) and CD45RA- CCR7- CD27- effector memory (EM; p=2.6e−06) CD8 T cells from allogeneic HSCT recipients compared with healthy controls. We did not observe any significant differences in CD57 expression in naïve nor in TEMRA CD8 T cells. Phenotypically, CD57+ CD8 T cells from allogeneic HSCT recipients displayed a senescent immunophenotype characterized by the low surface expression of CD127 that was further downregulated in CD57+ CD8 T cells from allogeneic HSCT recipients compared with healthy controls (p=0.00067). Functionally, CD57+ CD8 T cells from allogeneic HSCT recipients displayed a similar capacity to produce IFN-g, TNF-a, granzyme B and perforin when compared to CD57+ CD8 T cells from healthy controls. Virus-specific CD8 T cells identified upon stimulation with CMV, EBV, HHV6, BKV and Adenovirus peptides mainly displayed a CD27- effector memory phenotype in HSCT recipients (Figure 1C) and expressed higher levels of CD57 in HSCT recipients compared with healthy controls for CMV (p=0.017(; EBV p=0.018; Figure 1C-D). with a trend not reaching significance was for adenovirus, HHV6 and BK-virus-specific CD8 T cells. Using the replication of the non-pathogenic anellovirus TTV as a measure of impaired immunocompetence, we observed that the proportion of CD57-expressing cells among effector memory CD8 T cells positively correlated with TTV titers in allogeneic HSCT recipients (R=0.32, p=0.019; Figure 1E).
Conclusion: These results show that the proportion of phenotypically senescent CD57+ CD8 T cells increases after allogeneic HSCT as a result of an increased expression at the surface of memory CD8 T cells, including virus-specific cells. Moreover, CD57 expression at the surface of EM CD8 T cells, a highly enriched population in allogeneic HSCT recipients, correlated with higher replication of TTV, reflecting a status of impaired immunocompetence after allogeneic HSCT. Studies are ongoing to determine the utility of CD57 expression on T cells as a biomarker to predict infectious complications after allogeneic HSCT.
Chalandon: Incyte, BMS, Pfizer, Abbie, MSD, Roche, Novartis, Gilead, Amgen, Jazz, Astra Zenec: Other: Travel EXpenses, Accomodation; Incyte: Speakers Bureau; Incyte, BMS, Pfizer, Abbie, MSD, Roche, Novartis, Amgen: Other: Advisory Board. Simonetta: BMS Cellgene: Other: Ad-Hoc Advisory Board.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal